Aegis CHG-Impregnated Foam Disc Dressing
- Aegis chlorhexidine gluconate (CHG)-impregnated foam discs help reduce risk factors associated with CLABSI; absorbs exudate and releases CHG for up to seven days which helps shield the catheter insertion site while the CHG inhibits or kills bacteria on the dressing's surface
- Evidence-based practice standards recommend covering central line catheters with chlorhexidine-containing sponge dressings to help prevent central-line-associated bloodstream infections (CLABSI)1
- Radial slit allows easy placement around the catheter or drain site
- Indications for use: Absorbs exudate and covers the peri-wound area of a wound caused by vascular and non-vascular percutaneous medical devices
- Contraindications: CHG-impregnated foam dressings should not be used on premature infants or patients with a known sensitivity to chlorhexidine gluconate
- Convenient peel pouch
1 Marschall J, Mermel LA, Fakih M, Hadaway L, Kallen A, O'Grady NP, et al. Strategies to Prevent Central Line-Associated Bloodstream Infections in Acute Care Hospitals: 2014 Update. Infection Control and Hospital Epidemiology, Vol. 35, No. 7 (July 2014), pp. 753-771. Available at http://www.jstor.org/stable/10.1086/676533 Accessed September 10, 2015.
-
Hazardous: limited order quantity; no air shipments
-
Item Temperature Control
These products are part of a comprehensive CLABSI infection prevention system. Learn more.
For your business
To view pricing and availability
Ordering Information
Report Incorrect Product InformationTo see product options, make selections below.
| Diameter Inches |
|
| Hole |
|
| Latex Free |
|
| Product Type |
|
| UNSPSC |
|
Aegis is a hydrophilic foam disc impregnated with chlorhexidine gluconate (CHG) that supports clinical best practices and professional guidelines for reducing catheter-related bloodstream infections (CRBSI) and central line-associated bloodstream infections (CLABSI).2,3,6,7 It absorbs exudate and inhibits or kills microorganisms on the dressing’s surface.7
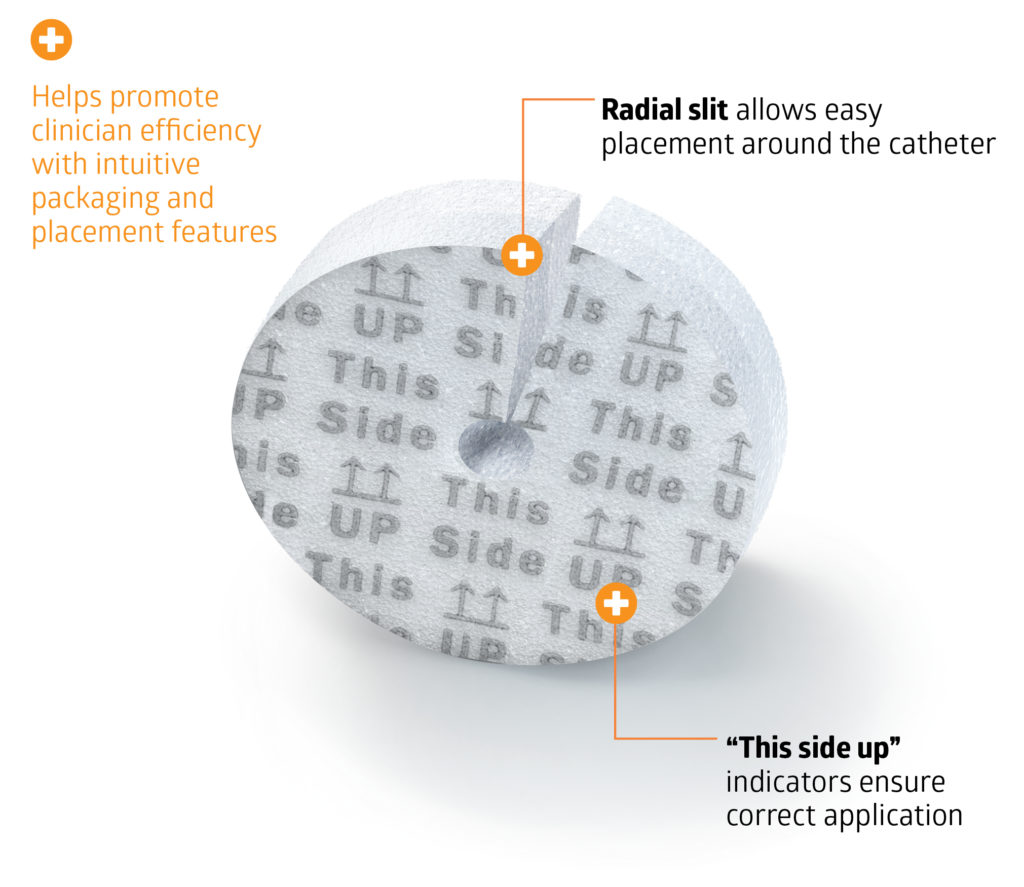
Experience the advantages of Aegis

Excellent absorbency
Absorbs up to 8x its weight in fluid with no distortion8

Fast kill rate
CHG begins killing bacteria within 2 hours7
Complete coverage
Provides 360° protection around the insertion site

Ongoing protection
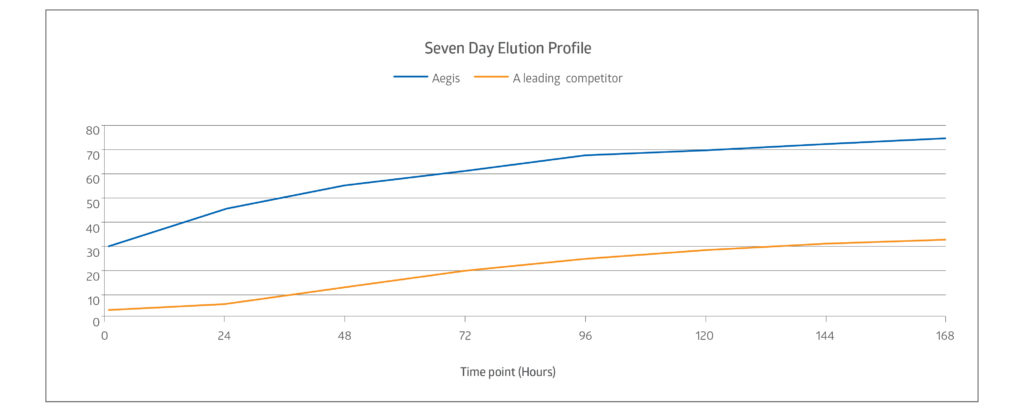
Sustains progressive “kill” over 7 days7
Indications for use*
Aegis is a CHG-impregnated foam dressing that absorbs exudate and covers the peri-wound area of a wound caused by vascular and non-vascular percutaneous medical devices, such as:
| AEG001S | AEG014S | AEG017S | |
|---|---|---|---|
| Size | 3/4″ disc (1.9 cm), 1.5 mm Hole | 1″ (2.5 cm), 4 mm Hole | 1″ disc (2.5 cm), 7 mm Hole |
| French size range | <6Fr | 6-12Fr | 13-20Fr |
| CHG content | 52.5 mg | 92 mg | 86.8 mg |
| Where to use |
|
|
|
| Pkg. | 10/bx, 4 bx/cs | 10/bx, 4 bx/cs | 10/bx, 4 bx/cs |
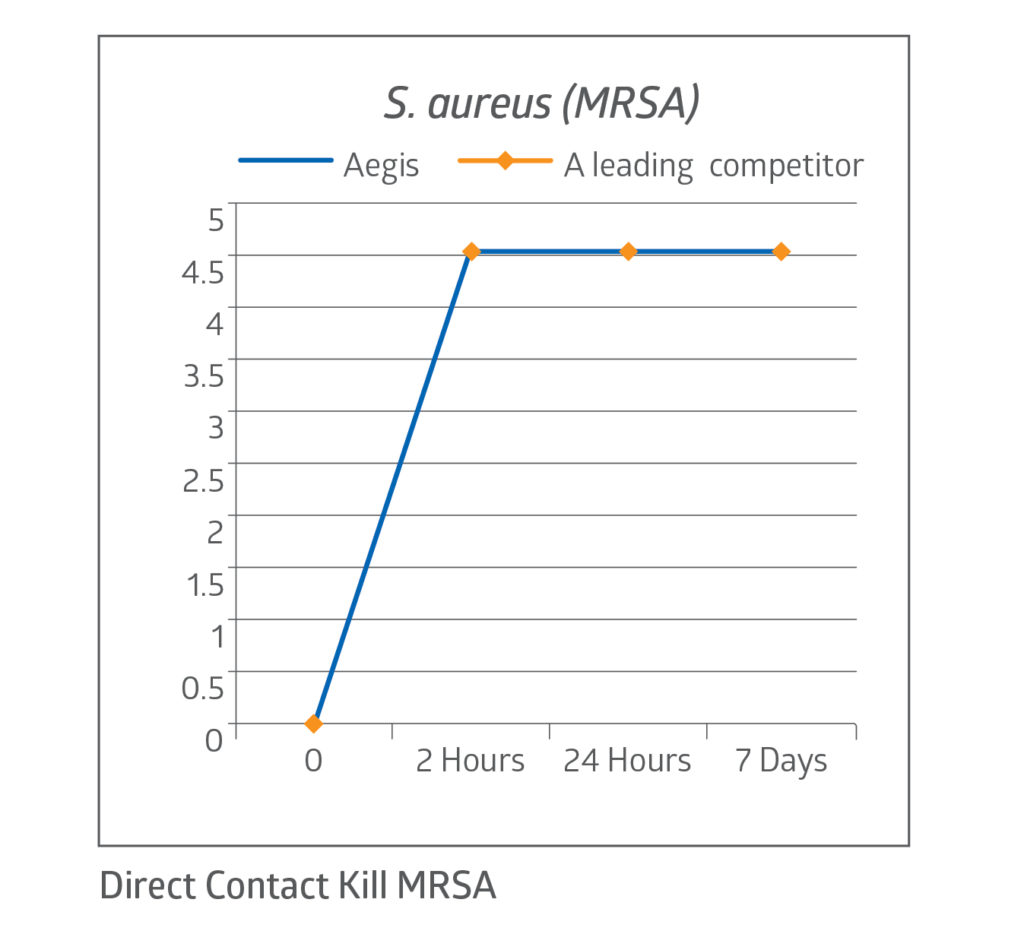
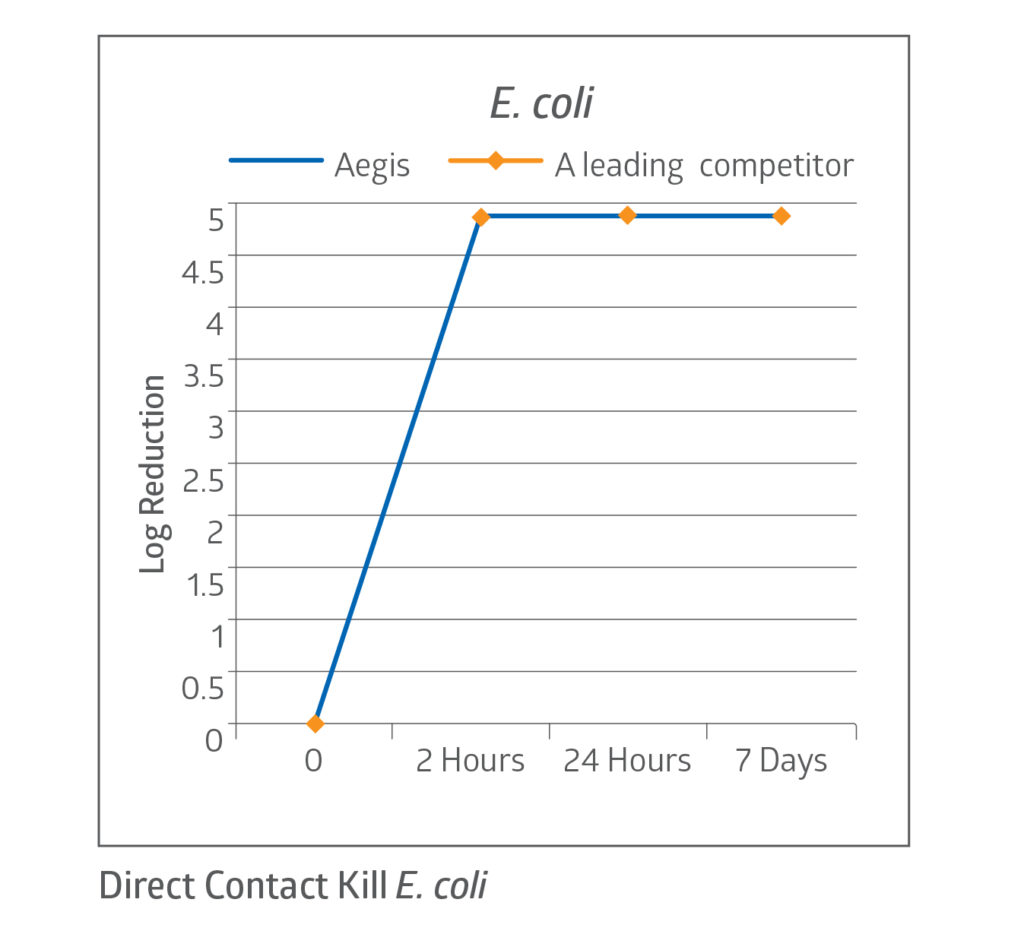
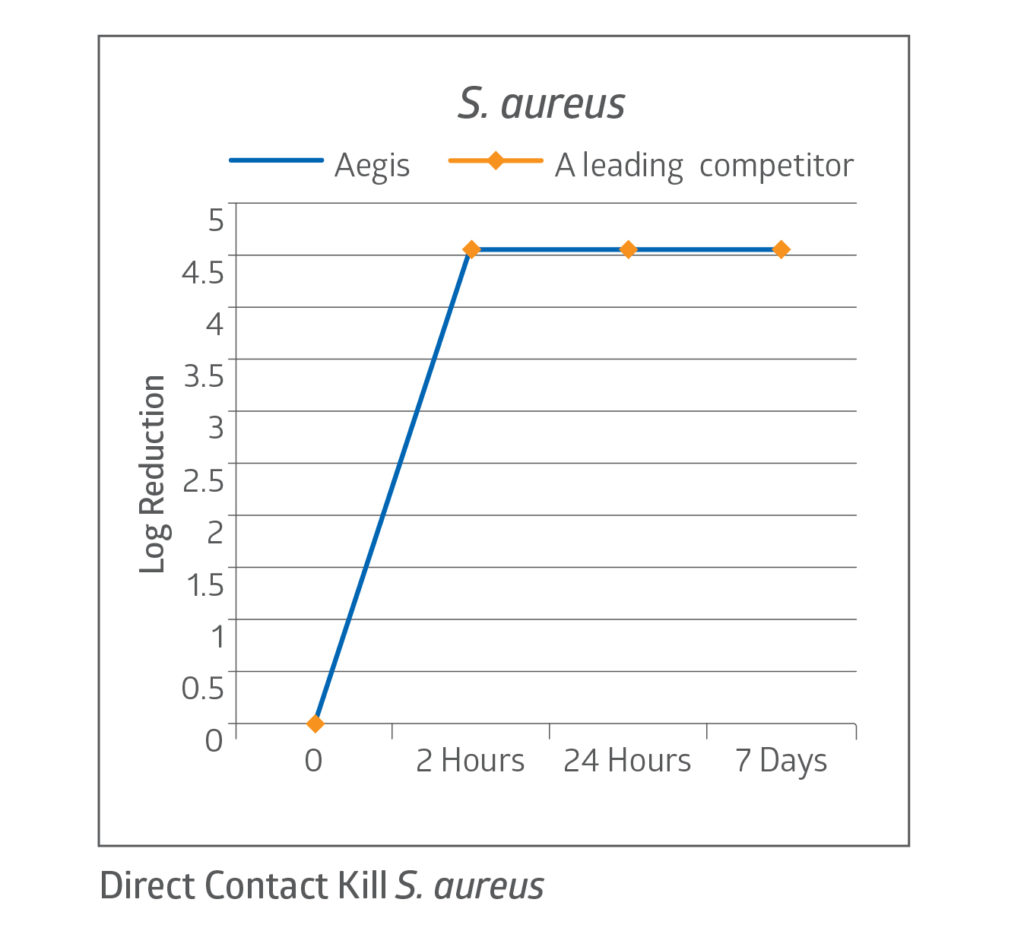
Fast kill rate
The CHG present in the dressing acts quickly, demonstrating a 4 log reduction in bacteria on the dressing’s surface within 2 hours.7
Excellent absorbency
The dressing’s two-layer foam absorbs up to eight times its weight in fluid with no distortion.8

Aegis product post fluid absorption

Leading competitor product post fluid absorption
References
- Hadaway LC. 5 Steps to Preventing Catheter-Related Bloodstream Infections. LPN2009. 2006;2(5):50-55. Available at: http://www.nursingcenter.com/ journalarticle?Article_ID=674810. Accessed December 21, 2016.
- Association for Professionals in Infection Control and Epidemiology. Guide to Preventing Central Line-Associated Bloodstream Infections. Washington, DC; APIC Implementation Guides, December 2015. Available at: http://www.apic.org/Professional-Practice/Implementationguides# Preventing. Accessed December 21, 2016.
- The Joint Commission. Preventing Central Line-Associated Bloodstream Infections: A Global Perspective. Oak Brook, IL: Joint Commission Resources, May 2012. Available at: https://www.jointcommission.org/assets/1/18/CLABSI_Monograph.pdf. Accessed December 21, 2016.
- Gahlot R, Nigam C, Kumar V, Yadav G, Anupurba S. Catheter-related bloodstream infections. International Journal of Critical Illness and Injury Science. 2014;4(2):162-167. Available at: https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC4093967/. Accessed June 6, 2017.
- Zimlichman E, Henderson D, Tamir O, et al. Health Care-Associated Infections: A Meta-Analysis of Costs and Financial Impact on the U.S. Health Care System. JAMA Intern Med. 2013;173(22):2039-2046. Available at: http://jamanetwork.com/journals/jamainternalmedicine/fullarticle/1733452. Accessed November 18, 2016.
- Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011. O’Grady NP, Alexander M, Burns LA, et al. Centers for Disease Control and Prevention website. Available at: http://www.cdc.gov/hicpac/BSI/BSI-guidelines-2011.html. Accessed November 18, 2016.
- Marschall J, Mermel LA, Fakih M, Hadaway L, Kallen A, O’Grady NP, Pettis AM, Rupp ME, Sandora T, Maragakis LL, Yokoe DS. Strategies to Prevent Central Line-Associated Bloodstream Infections in Acute Care Hospitals: 2014 Update. Infection Control and Hospital Epidemiology, Vol. 35, No. 7 (July 2014), pp. 753-771. Available at http://www.jstor.org/stable/10.1086/676533. Accessed November 18, 2016.
- Data available upon request

